
The DENALI Phase 2 study is evaluating a first-in-class WEE1 inhibitor as a potential treatment for platinum-resistant ovarian cancer (PROC).
Below, healthcare professionals can find key details to help evaluate trial eligibility, understand the investigational therapy, and guide conversations with patients.
This study is enrolling people with ovarian cancer that has recurred within six months of completing platinum-based chemotherapy. Approximately 50% of people with PROC may be eligible.
The trial enrollment is biomarker-driven using available tumor samples that will be tested on the clinical trial diagnostic test to confirm eligibility.
Consider submitting eligible patient samples for Cyclin E1 testing now, even while patients are on current therapy, to ensure rapid identification upon disease progression.
See inclusion criteria below:
Age 18+
Participants must have ovarian cancer that has recurred within six months of completing platinum-based chemotherapy
Tumor testing must confirm a positive Cyclin E1 protein overexpression*
Prior therapy:
The DENALI Phase 2 study therapy is the first and only WEE1 inhibitor being evaluated for PROC in a potentially registrational-enabling trial, a breakthrough that is exciting for many in the scientific community.
The therapy is administered orally and is designed to target cancer based on specific tumor characteristics identified through Cyclin E1 biomarker testing using a companion diagnostic.
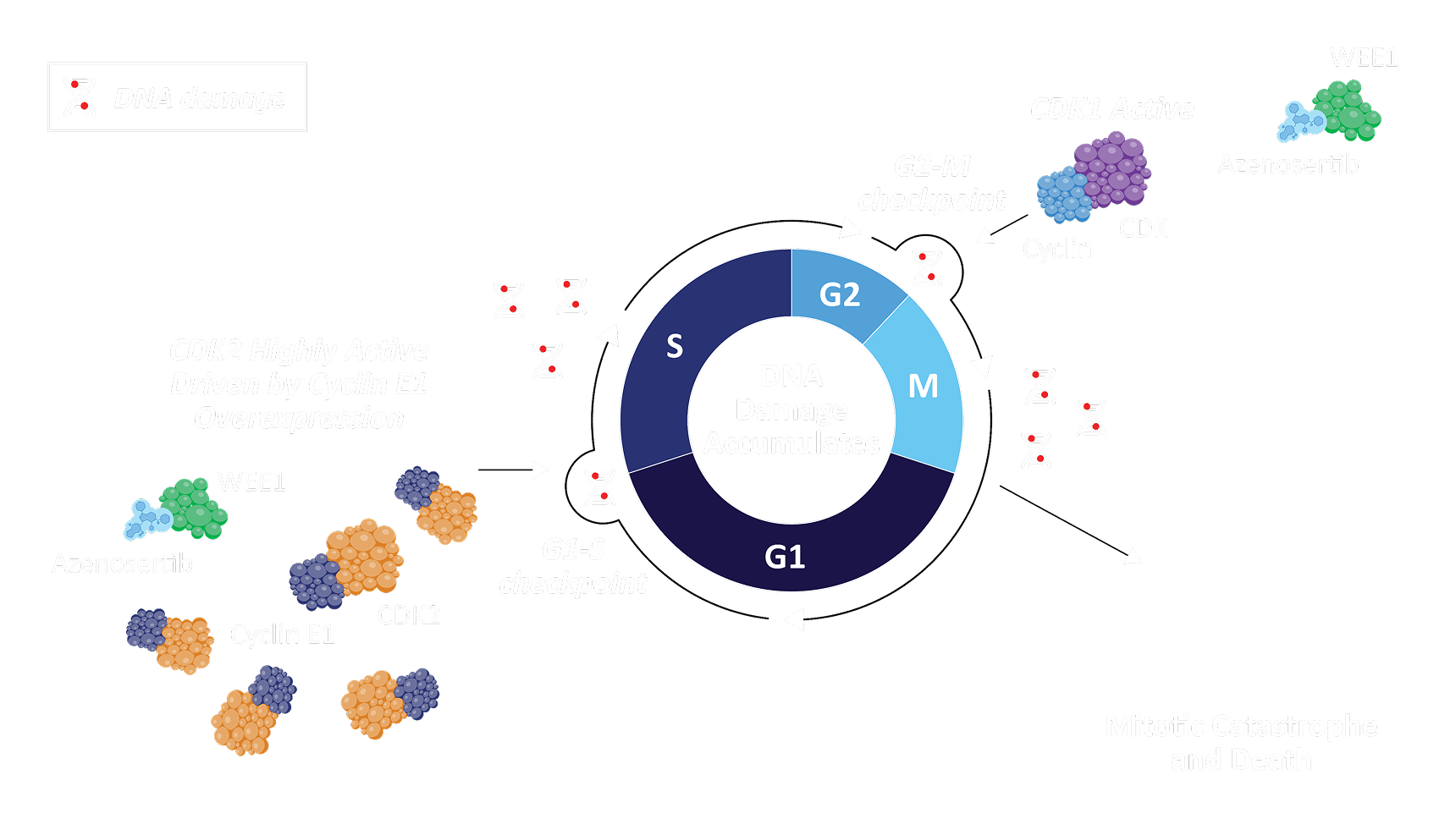
WEE1 is a tyrosine kinase with a key role in several stages of the cell cycle, including the G1‑S and G2‑M checkpoints through negative regulation of both CDK1 / 2, preventing replication of cells with damaged DNA¹ ².
WEE1 inhibition results in unscheduled mitosis without adequate DNA repair and eventual cancer cell death¹ ³.
The investigational therapy is administered orally, allowing patients to take the medication at home as part of their routine care.
There is no placebo arm in this Phase 2 trial. All participants receive active therapy at one of two dose levels: 300mg or 400mg.
No additional biopsy is required, as biomarker testing relies on previously collected tissue samples.
With patient consent, physicians should submit previously collected tumor samples for testing with the study’s companion diagnostic to identify patients who may be eligible to participate.
Participants will receive care from exceptional clinical teams at leading academic and research institutions across the country.

This information may help your patient feel more confident in discussing whether the DENALI study is an appropriate next step in their treatment journey.