
The Phase 2 DENALI study is evaluating a new, investigational medicine to treat platinum-resistant ovarian cancer (PROC).
For those facing the challenges of PROC, new options are urgently needed. This trial uses biomarker testing to match this oral therapy to the people most likely to benefit—and approximately 50% of people with PROC may be eligible.
This oral therapy could potentially shift intensive infusions into a more convenient, manageable routine. "Investigational" means that the study treatment has not been approved for use for PROC, except in clinical trials. What we learn from this study may help scientists and doctors develop the study treatment as a possible treatment for PROC.
This trial is designed to fit more easily into your life:
Oral medication delivery means treatment is taken at home, making it less intrusive and easier to integrate into daily life.
Every participant in this dosing trial receives active therapy; there is no chance of receiving a placebo. The study is designed to compare different dosing regimens to determine the optimal balance of safety, tolerability, and therapeutic benefit for patients.
The study is conducted at top institutions across the country, providing access to clinical teams and an opportunity to contribute to scientific discovery.

The DENALI study is evaluating a first of its kind, small molecule, highly selective oral WEE1 kinase inhibitor.
Eligibility is determined by Cyclin E1 protein expression, as assessed by the clinical trial diagnostic test, which also helps identify patients most likely to experience benefits from the treatment.
This expands upon the eligibility criteria of Phase 1, which was limited to CCNE1 amplified patients, opening the door to more patients who may benefit from this investigational therapy.
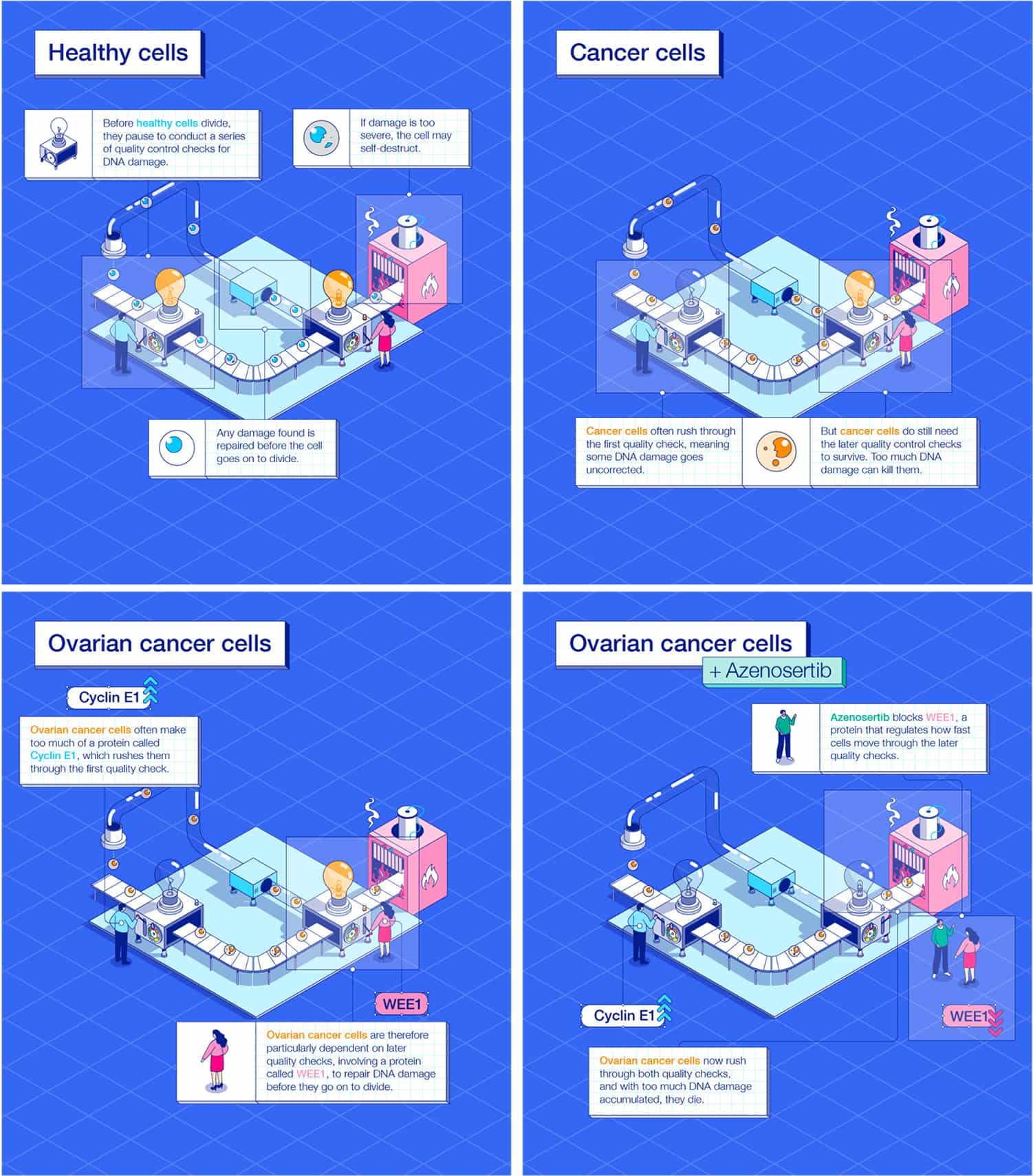
Before healthy cells divide, they normally pause to conduct a series of quality control checks for DNA damage. If damage is found, the cell may undergo repairs.
Cancer cells often rush through the first quality check, meaning some DNA damage goes uncorrected. That damage is one reason cancer cells grow so fast.
But cancer cells do still need the later quality control checks to survive. Too much DNA damage can cause cancer cells to die.
Ovarian cancer cells often make too much of a protein called Cyclin E1, which moves them through the first quality check even faster, making DNA damage more likely. Ovarian cancer cells are therefore particularly dependent on the later quality checks to survive.
WEE1 is a protein that regulates how fast cells move through the later quality checks. Because ovarian cancer cells are already neglecting the first quality check, rushing through the later quality checks may kill cancer cells.
Medicines that inhibit WEE1 are believed to be effective at killing cancer cells, ovarian cancer in particular.

Wondering if the DENALI trial is right for you? You may qualify if:
You have ovarian cancer, which has recurred within 6 months of finishing chemotherapy. This is called platinum-resistant ovarian cancer.
You have an over-expression of a protein called Cyclin E1 in your body.
Use our site locator to quickly identify participating DENALI clinical trial locations in your area.